The 2025 Medical Device Nonvisual Accessibility Act: What Low Vision Americans Need to Know + Zoomax Solutions

Abstract
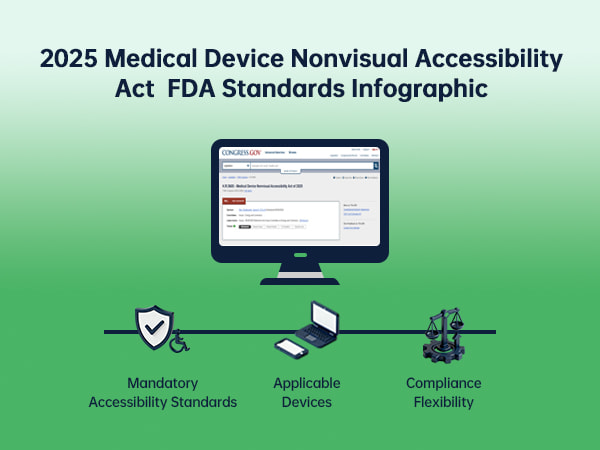
The 2025 Medical Device Nonvisual Accessibility Act (H.R.5605) marks a pivotal step in advancing healthcare equity for low vision and blind Americans. This legislation mandates the FDA to establish accessibility standards for Class II and III home medical devices with digital interfaces, ensuring nonvisual access to critical health information. For the over 7 million low vision individuals in the U.S., the act transforms independent health management by reducing reliance on caregivers. This article deciphers the act’s core requirements, its real-world impact, and how Zoomax’s electronic magnifiers serve as immediate, compliant solutions to bridge current accessibility gaps, empowering users to engage with medical devices autonomously.
1. Introduction: The Urgency of Nonvisual Access in Home Healthcare
 Over 7 million low vision Americans and 1 million blind individuals face a daily barrier: navigating home medical devices designed primarily for visual users. From reading blood pressure monitors to programming insulin pumps, these tasks often require reliance on family members or caregivers, compromising privacy, dignity, and timely health management. In September 2025, the U.S. House of Representatives introduced the Medical Device Nonvisual Accessibility Act (H.R.5605), a landmark bill aimed at addressing this inequity. By mandating the FDA to create accessibility standards for key home medical devices, the act signals a new era of inclusive healthcare. This article explores the act’s provisions, its impact on low vision communities, and how Zoomax’s electronic assistive technology aligns with its goals to enhance independent living.
Over 7 million low vision Americans and 1 million blind individuals face a daily barrier: navigating home medical devices designed primarily for visual users. From reading blood pressure monitors to programming insulin pumps, these tasks often require reliance on family members or caregivers, compromising privacy, dignity, and timely health management. In September 2025, the U.S. House of Representatives introduced the Medical Device Nonvisual Accessibility Act (H.R.5605), a landmark bill aimed at addressing this inequity. By mandating the FDA to create accessibility standards for key home medical devices, the act signals a new era of inclusive healthcare. This article explores the act’s provisions, its impact on low vision communities, and how Zoomax’s electronic assistive technology aligns with its goals to enhance independent living.
2. Decoding the Act: Core Requirements and Scope
The 2025 Medical Device Nonvisual Accessibility Act centers on three critical pillars to ensure equitable access:
Mandatory Accessibility Standards
The FDA is tasked with developing regulations that require Class II and III home medical devices with digital interfaces to provide nonvisual access equivalent to visual access. This includes features like screen readers, tactile feedback, or voice guidance to enable users to operate devices, read data, and access menus independently.
Applicable Devices
The act covers common home medical equipment such as blood glucose monitors, continuous positive airway pressure (CPAP) machines, home chemotherapy infusion pumps, and blood pressure monitors. It excludes devices used exclusively by healthcare providers or those without digital interfaces to avoid overburdening manufacturers.
Compliance Flexibility
Manufacturers may request exemptions if meeting standards would “fundamentally alter the device’s purpose” or impose “unreasonable economic burdens.” However, the FDA must publicly justify all exemptions, ensuring transparency and accountability to the disability community.
3. How the Act Transforms Life for Low Vision Americans
For low vision individuals, the act is more than legislation—it’s a gateway to autonomy:
Independent Health Management
No longer will users need to ask others to read device displays or adjust settings. With accessible features, they can monitor chronic conditions, administer medications, and track health data without assistance, reducing errors and improving treatment adherence.
Enhanced Privacy and Dignity
Health information is personal, and the act eliminates the need to share sensitive data with caregivers, empowering users to maintain control over their healthcare journey.
Alignment with ADA Principles
The act builds on the Americans with Disabilities Act (ADA) by extending accessibility requirements to home medical devices, closing a longstanding gap in healthcare inclusivity.
Short-Term Challenges
While the act is transformative, FDA standard development and manufacturer compliance will take time. In the interim, many low vision users will still face barriers with older devices—creating a critical need for immediate solutions.
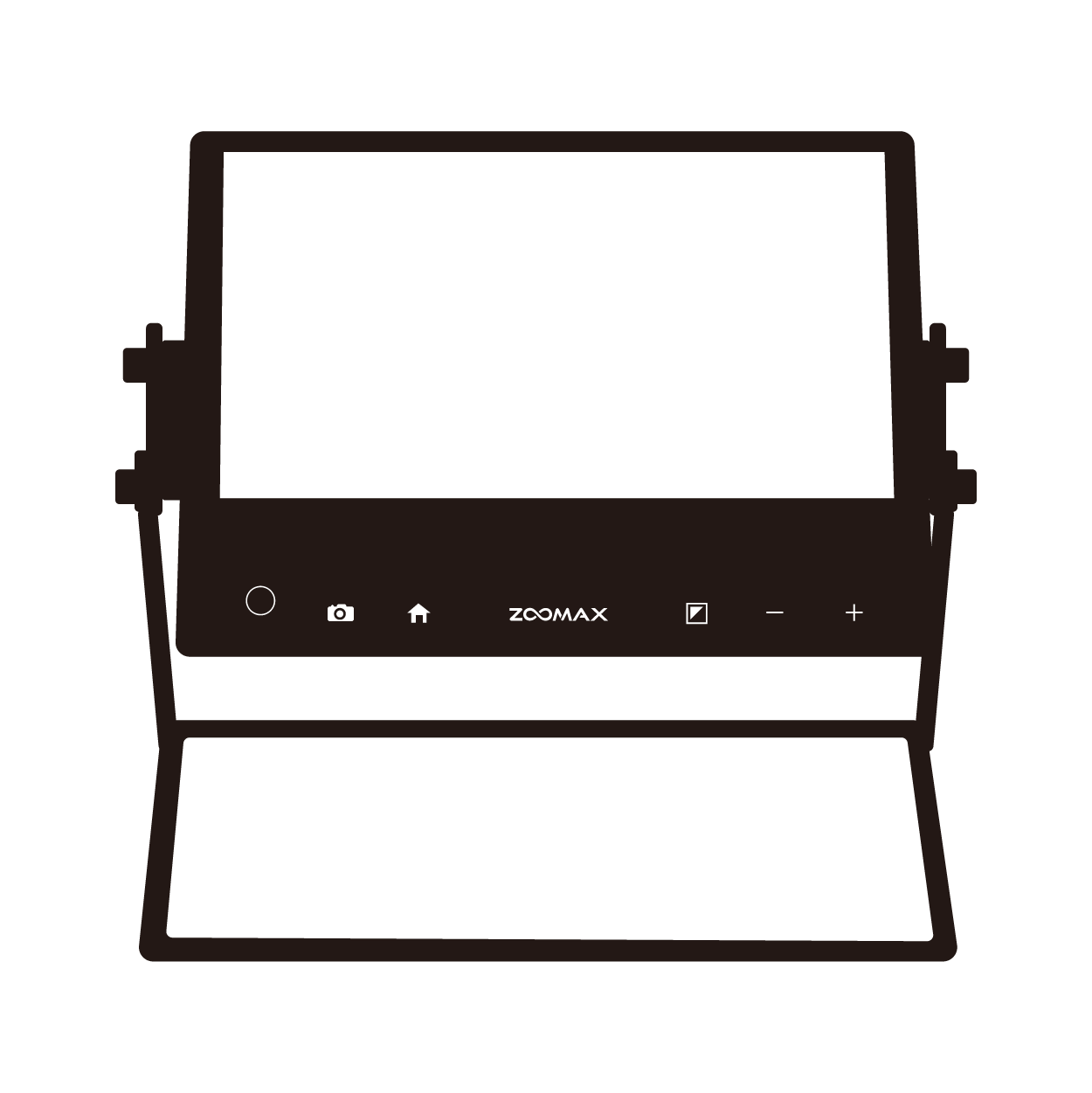
4. Zoomax Electronic Magnifiers: Bridging the Accessibility Gap
Zoomax, a global leader in low vision assistive technology, offers electronic magnifiers that directly address the act’s goals, serving as both interim and long-term solutions:
Core Features for Medical Device Accessibility
Zoomax’s devices are engineered to solve the specific challenges of reading medical device interfaces:
- High-Definition Magnification: Models like the Zoomax low vision magnifiers offer 1–32x zoom, enabling users to enlarge small digital displays, buttons, and text on blood pressure monitors, insulin pumps, and other equipment.
- Contrast and Color Adjustments: Features like black-white reversal and high-contrast modes enhance readability, even for users with severe visual impairments.
- Portable and Versatile Designs: Handheld (Luna electronic magnifier series) and wearable (Acesight VR) options fit seamlessly into home healthcare routines, allowing users to access devices anywhere, anytime.
- Voice Guidance Integration: Select Zoomax models include text-to-speech function which reads aloud medical data (e.g., “Blood pressure: 120/80”) for complete nonvisual access—aligning perfectly with the act’s nonvisual requirements.
Long-Term Synergy with Compliant Devices
As manufacturers adopt FDA standards, Zoomax’s magnifiers will complement new accessible devices, providing an extra layer of support for users who prefer visual magnification alongside built-in accessibility features.
5. Future Outlook: Accessibility as a Healthcare Priority
The 2025 Medical Device Nonvisual Accessibility Act is a catalyst for industry-wide change. Manufacturers will increasingly integrate accessibility into product design, driving innovation in features like Bluetooth connectivity for voice assistants and haptic feedback. Zoomax remains at the forefront of this shift, with plans to enhance device interoperability—enabling direct syncing with home medical equipment to auto-magnify displays and broadcast data. For low vision Americans, this means a future where healthcare technology adapts to their needs, not the other way around.
6. Conclusion: Empowering Independence Through Inclusive Policy and Technology
The 2025 Medical Device Nonvisual Accessibility Act is a milestone for healthcare equity, recognizing that access to medical information is a fundamental right, not a privilege. For low vision Americans, it promises greater independence, privacy, and control over their health. While policy progress is critical, immediate solutions like Zoomax’s electronic magnifiers ensure that users don’t have to wait for a more inclusive future—they can access the support they need today. As the FDA develops standards and manufacturers comply, collaboration between policymakers, tech providers like Zoomax, and the low vision community will be key to building a healthcare system that leaves no one behind.
To learn how Zoomax’s electronic magnifiers can enhance your ability to use home medical devices, visit our website at www.zoomaxusa.com or contact a local U.S. dealer for a personalized demonstration.